MicroRNAs (miRNAs):
Function: miRNAs are small RNA molecules that play a crucial role in the regulation of gene expression. Their presence and specific levels in urine can serve as indicators of various pathologies — particularly bladder cancer.
Detection: The BlaDimiR system focuses on detecting and quantifying specific miRNAs whose presence in urine samples is indicative of a tumoral process. Similarly, using distinct biomarker sets, the presence of certain miRNAs can indicate a molecular status associated with non-response to BCG therapy (BlaDimiRplus) and/or to combined BCG and anti-PD-L1 therapy (BlaDimiRIO).
Diagnostic / Predictive Process:
Sample Collection: A urine sample is collected from the patient through a standard, non-invasive procedure.
miRNA Analysis: Urine samples are analyzed in a laboratory using real-time polymerase chain reaction (qPCR). This technique enables the precise quantification of specific miRNAs.
qPCR: qPCR is a standard molecular technique used in most laboratories to amplify and quantify RNA, providing rapid and accurate results on the presence of miRNAs associated with bladder cancer.
Result Interpretation:
Sensitivity and Specificity: BlaDimiR has demonstrated a sensitivity of 90.5% and a specificity of 97%, achieving an overall diagnostic accuracy of 96% for bladder cancer detection.
Likewise, BlaDimiRplus can predict response to BCG therapy in 91% of cases, with 85.7% sensitivity and 92.3% specificity.
In the case of BlaDimiRIO, our laboratory results indicate a predictive accuracy of 85%, combining miRNA measurement with cytokine profiling, with 75% sensitivity and 91% specificity.Results: The qPCR analysis provides quantitative data indicating whether miRNA levels are consistent with the presence of bladder cancer, and whether they predict response to BCG or combined BCG + anti-PD-L1 treatments.
Technological Advantages:
Non-invasive: Unlike cystoscopy, which is painful and invasive, BlaDimiR relies solely on a urine sample — reducing patient discomfort and enabling remote monitoring.
Fast and Accurate: qPCR technology delivers rapid, high-precision results, improving both detection and follow-up of bladder cancer. It also provides clinicians with a robust scientific basis to guide therapeutic decisions according to each patient’s molecular profile.
BlaDimiR Technology
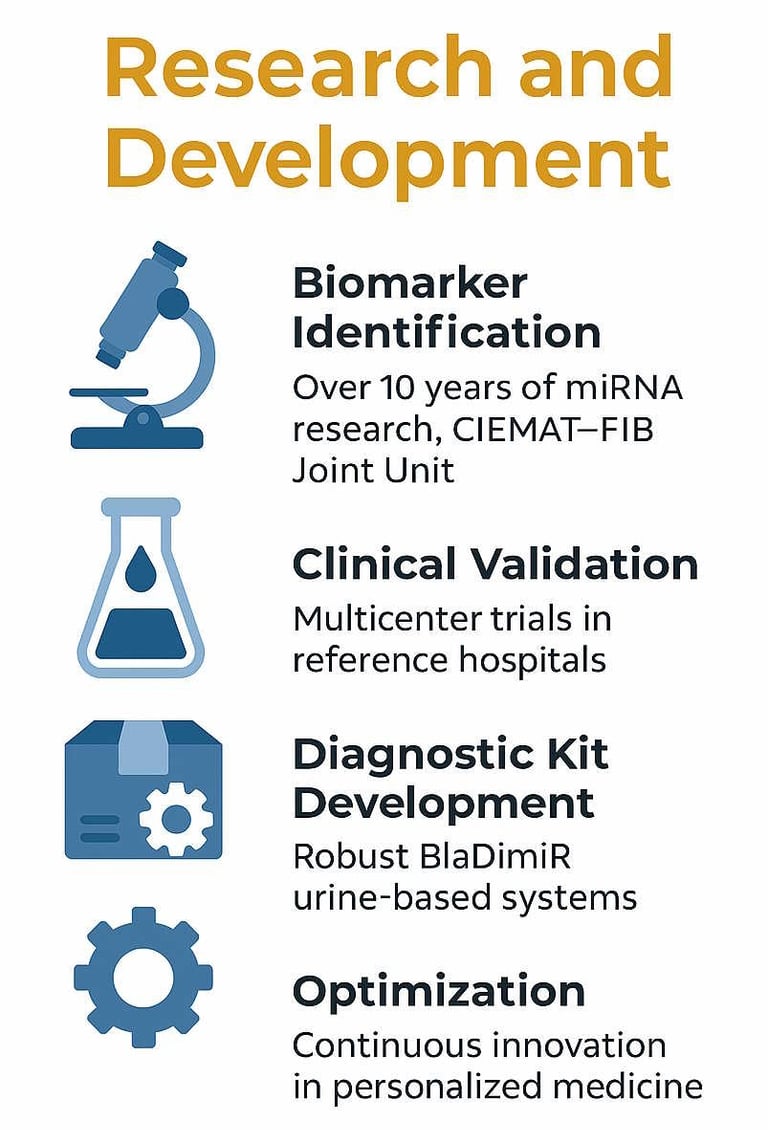
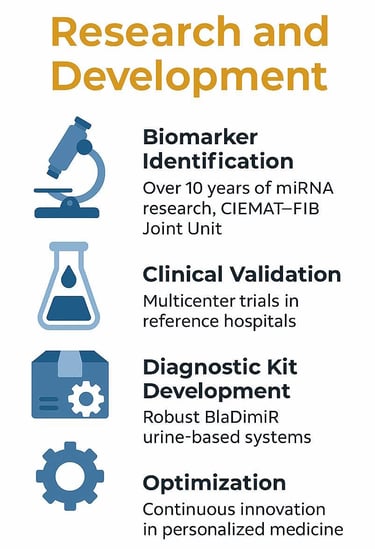
Biomarker Identification: The identification of specific miRNAs for diagnosis and precision medicine based on urine samples is the result of more than a decade of research conducted by the Joint CIEMAT-FIB Unit at Hospital 12 de Octubre in Madrid.
The scientific results supporting these robust systems, known as BlaDimiR, have been presented at multiple scientific congresses (link) and published in peer-reviewed journals (link).
In these studies, we demonstrated that miRNAs are robust, stable, and reproducible markers in urine samples, and that urine can provide the same biochemical information as tissue biopsy samples.
Clinical Validation: The three components of the BlaDimiR System have been evaluated across different sample cohorts considered as discovery (first sample set), proof of concept (second sample set), and validation (third sample set).
After successfully completing these three phases, the system is now undergoing a multicenter clinical trial in Spain, currently in the recruitment phase, with the participation of six national reference hospitals.
Diagnostic Kit Development: Based on the research outcomes and through a process of scientific rigor, all necessary quality control, stability, and reproducibility studies of every component of the kit have been performed.
The resulting documentation is being prepared for submission to the Regulatory Authority to obtain approval for the commercialization of these diagnostic systems.
Optimization: The BlaDimiR system is under continuous research and development to further enhance its diagnostic and predictive accuracy.
In parallel, new biomarkers are being incorporated to support emerging therapeutic strategies for bladder cancer patients, reinforcing the system’s role in personalized medicine.
Research and Development
Scientific Collaborations:
Carvalho S, Abreu CM, Ferreira D, Lima L, Ferreira JA, Santos LL, Ribeiro R, Grenha V,
Martínez-Fernández M, Duenas M, Suárez-Cabrera C, Paramio JM, Diéguez L, Freitas PP, Oliveira MI.
Phenotypic Analysis of Urothelial Exfoliated Cells in Bladder Cancer via Microfluidic Immunoassays:
Sialyl-Tn as a Novel Biomarker in Liquid Biopsies.
Frontiers in Oncology, 2020 Sep 16; 10:1774.
https://doi.org/10.3389/fonc.2020.01774
PMID: 33042825






